
Generally, galvanic action occurs where different levels of corrosions protection are in contact and the hierarchy of corrosion protection will influence the overall performance. Lower level corrosion protected elements will ‘sacrifice’ themselves ahead of higher protected materials they are in contact with. As a result, dissimilar metals (such as Type 316/304 stainless steel and zinc) in contact with each other and located in a corrosive environment will exhibit an increase in the rate of corrosion activity, compared to the rate of corrosion activity that would ordinarily occur for similar protected materials in contact. The increase in the rate of corrosion activity is affected by many factors, such as the specific dissimilar metals and the local environment. BRANZ has investigated galvanic action and more information can be found on their website.
In an effort to better understand the corrosion effect particularly of combining stainless steel fasteners with zinc protected products, Simpson Strong-Tie also conducted a controlled corrosion test in a salt-spray environmental chamber (ASTM B117). The test consisted of a zinc–coated washer and zinc coated and powder-coated post bases assembled with both zinc-coated Titen HDs (the control samples) and with stainless steel Titen HDs (the subject samples). After 1000 hours of salt spray, the corrosion amounts were measured. The results of this test showed that the corrosion rate for these assemblies was not increased when the dissimilar metals of the stainless-steel Titen HD and zinc-coated post base were in direct contact. An important note is that other dissimilar metal assemblies will not perform the same under similar conditions.
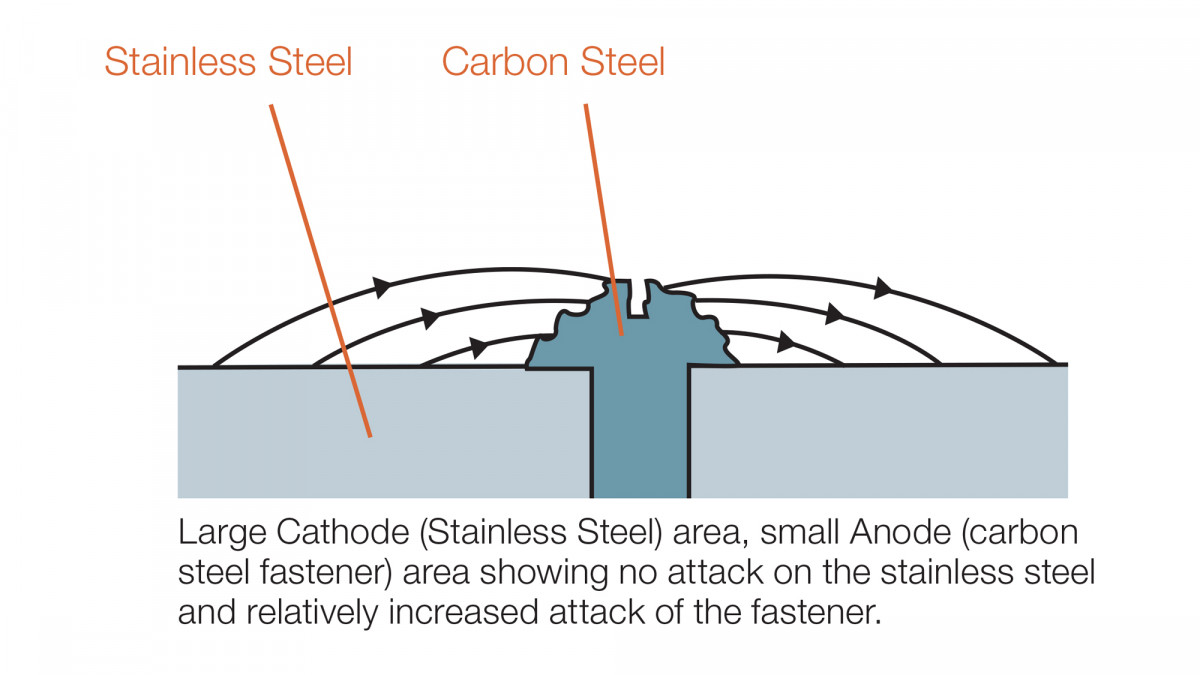
Surface areas of the dissimilar metals contribute significantly to the overall performance, and so using a large zinc protected steel elements with stainless fasteners for example would be expected to perform reasonably well. Whereas a large stainless connector fixed with zinc protected fasteners will result in significantly accelerated corrosion of the fasteners. This is due to the galvanic action by which the zinc on the fasteners will ‘sacrifice’ itself to ‘protect’ the surrounding stainless. Because of the small amount of zinc present on the fasteners, this would quickly be consumed and the black steel left to corrode.
It is particularly important therefore to use stainless fasteners with stainless (or potentially zinc protected) connectors, and zinc protected fasteners avoided with stainless connectors unless a proper study to understand the effects has been undertaken.
Article by Daniel Scheibmair (Structural Engineer)











 New Products
New Products

















 Popular Products from Simpson Strong-Tie
Popular Products from Simpson Strong-Tie


 Most Popular
Most Popular


 Popular Blog Posts
Popular Blog Posts
